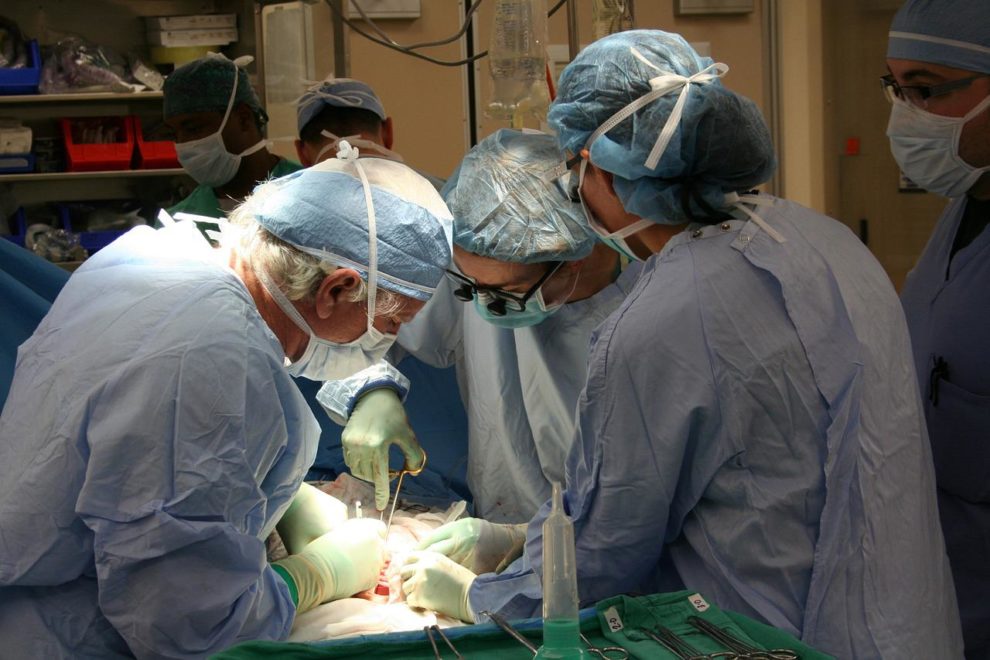
According to media reports, the FDA’s advisory committee has deliberated on how to proceed with allowing clinical trials to test pig-to-human organ transplants.
The xenotransplantation procedure dates back to the 17th century, when attempts were made to use animal blood for transfusions. Due to a scarcity of human organs, scientists began looking at non-human primates such as monkeys, chimps, baboons, and even pigs, according to Nature.
Pig experiments yielded more positive results because their organs are comparable to those of humans.
While a few such procedures have been carried out in the last year, there have been no formal human xenotransplant trials.
According to the report, most agency officials and physicians agree that human trials are required to help answer the most pressing research questions about interspecies transplants.
There is also a need for information on the pig breed best suited for growing transplant organs, as well as how co-occurring health conditions, such as diabetes, may affect transplantation success.
“Human trials would help to answer a slew of questions, including what is the best cocktail of immunosuppressive drugs to give humans to help their bodies accept a pig organ, and how can physicians manage the risk that transplanted organs might harbour a pig virus,” added Caroline Zeiss, a veterinary specialist at Yale University.
The growing number of people waiting for organ transplants adds to the urgency of the trials. More than 100,000 people in the United States alone are waiting for organ transplants. Long have researchers hoped that xenotransplantation could help meet demand and thus save lives.
“We have people dying each day waiting for organs,” According to Jay Fishman, a transplant infectious disease specialist at Massachusetts General Hospital in Boston who attended the FDA meeting.
A study published in the American Journal of Transplantation in September 2021 demonstrated the transplantation of two kidneys from a gene-edited pig onto a brain dead patient. During the 54-hour test, the kidneys appeared to function normally and produce urine.
A similar surgery was performed in October by doctors at NYU Langone Health in New York.
In January of this year, a severely ill man in Maryland, US, became the first to receive a pig heart. He died two months later, however, from a porcine virus, a preventable infection linked to devastating effects on transplants.
Even though the heart transplant recipient died, Allan Kirk, a transplant surgeon at Duke University in the United States, was quoted as saying that the surgery was a huge success.
The high-profile transplants have “increased public awareness of the field,” Wilson Bryan, director of the FDA’s Office of Tissues and Advanced Therapies in Silver Spring, Maryland, said at the meeting.
United Therapeutics-owned companies such as Revivicor in Blacksburg, Virginia, have been breeding pigs for use in xenotransplantation.
According to the report, they have been looking for the right combination of genetic modifications for their pigs to help ensure that human immune systems accept organs from the animals.